TECHNIQUES FOR PRESERVING LIFE CYCLE
STAGES
SAVING, STORING AND PRESERVING OOCYSTS
FOR OBSERVATION
Before oocysts can be studied
critically, they must be properly maintained to keep them viable so that
their structural integrity remains intact. in our experience, oocysts
from different vertebrate host species fall into two groups, which, of
necessity, need to be handled differently when collected under field
conditions.
- Oocysts from birds, mammals, and terrestrial
invertebrates and
reptiles
These oocysts keep best when fresh feces
are placed directly in 2-2.5% aqueous (w/v) potassium dichromate
(K2Cr2O7) in a ratio of 1 volume of
feces : greater than or equal to 5 volumes postassium dichromate. In
field
collections, either snap-cap or screw-cap 16-25 ml vials work well, but
one should not fill the vial all the way to the top; leave a layer of
air between the top of the feces-dichromate mixture and the cap to allow
the oocysts some atmospheric oxygen. Unfortunately, other solutions for
feces, for example, 2% aqueous sulfuric acid (see Wash et al.,
1985) or common laboratory fixatives for oocysts (see Duszynski and
Gardner, 1991) have proven unsatisfactory either for keeping oocysts
viable or for preserving them as types.
- Oocysts from amphibians, fish and aquatic
invertbrates and reptiles
These oocysts often are very thin-walled
and fragile and sometimes prove difficult to sporulate. When examining
hosts from freshwater environments, fresh mucus and feces from the
intestinal tract should be placed in vials with tap water or with
filtered river water at room temperature. Likewise, mucus and gut
contents of marine animals should be placed in containers with filtered
seawater. These fecal-water solutions must be supplemented with 200 IU
penicillin g/ml, 200 ug streptomycin/ml, and 0.5 ug Fugizone/ml (see
Upton et al., 1988; Molnár, 1996).
Upon return to the laboratory, the
fecal-dichromate or fecal-water-antibiotic mixtures should be placed
into a petri dish, any fecal pellets should be broken up, and the fecal
material spread out in the dish and covered (Duszynski and Conder,
1977). The petri dishes generally should be maintained at room
temperature (20-23 º C) for 7-10 days, which will allow any oocysts
present to sporulate. Fecal-dichromate mixtures (terrestrial hosts)
should not be refrigerated prior to the sporulation process as, in our
experience, this will interfere with sporulation success. However,
oocysts of some marine fishes were found to sporulate adequately only
when the fecal-supplemented seawater mixture was placed on ice for 7-8
days (Upton et al., 1988); in this instance, the oocyst wall ruptured
shortly after sporulation, releasing free sporocysts. In most species,
however, after about 7-10 days, the mixture can be washed from the petri
dish with clean potassium dichromate into a screw-cap jar (disposable
baby bottle jars work well) filled only about half way and then put into
a standard refrigerator (4-7 º C) until the material can be
examined (sugar flotation) for the presence of oocysts. In our
experience, oocysts of terrestrial vertebrates can remain viable, or at
least stucturally intact, in the refrigerator for 3-4 years, whereas
oocysts of certain fish coccidia (Upton et al., 1988, Molnár, 1996)
may deteriorate soon after sporulation and die within a few days or
weeks. Thus, it is probably best to study and document the structure of
sporulated oocysts as soon as possible after they are sporulated.
Sporulated oocysts are best separated
from the dichromate-fecal mixture by suspending an aliquot (1-3 ml) from
the sample in 14-12 ml of modified Sheather's (Sheather, 1923) sugar
flotation solution (500 g sucrose, 350 ml tap water, 5 ml phenol) via
centrifugation (5 min at 2,000 rpm). It is important to use only number
1, 18 mm2 coverslips on top of the 15 ml centrifuge tubes
(those with a smooth, beaded edge work best) as this reduces the surface
area that needs to be scanned for oocysts. After centrifugation, lift
the coverslip carefully from the centrifuge tube, place onto a glass
slide, and set aside for 5-10 minutes; this allows the sugar along the
edges of the coverslip to harden and minimizes movement of the oocysts
during observation, measurement, and photography. The coverslip should
be scanned systematically (100-400x total magnification) until oocysts
are located.
Measuring and detailing the structure of sporulated oocysts should
always be done only under an oil immersion objective (Neofluor and
Nomarski optics are both useful). Apochromatic lenses are superior to
achromats and the higher the numerical aperture on the objective lens,
the more accurate will be the measurements.
GUIDELINES FOR DESCRIPTIONS AND SPECIES
DIFFERENTIATION
We strongly suggest that the following
criteria be presented to allow accurate evaluation of a proposed new
species description for coccidia (family Eimeriidae). In the list of
features below, we have followed the example of Lom and Arthur (1989) by
marking those features that are indispensable with a solid circle, while
those recommended for inclusion are marked with
an open circle.
The Host
- Make sure that the host has been reliably identified
by a knowledgable taxonomist who works with the host group and use the
most up-to-date scientific name and its authority for the species.
- Host life stage infected (larva, juvenile, adult); this
may be more
important for some host groups (e.g., fish) than for others (e.g.,
mammals).
- Locality(ies) where infected hosts were collected; supply
GIS coordinates whenever possible.
- Prevalence of infection by locality; include seasonal
prevalences, if possible.
- Whenever possible, deposit the actual host specimen from
which the new species was described (=symbiotype specimen, see Frey et
al., 1992) into an appropriate, accredited museum.
- Give any ecological data, habitat data, or host genetic
data that may seem relevant (for examples, see Couch et al., 1993;
Wilber, Hanelt, et al., 1994; Wilber, McBee, et al., 1994).
The Sporulated Oocyst
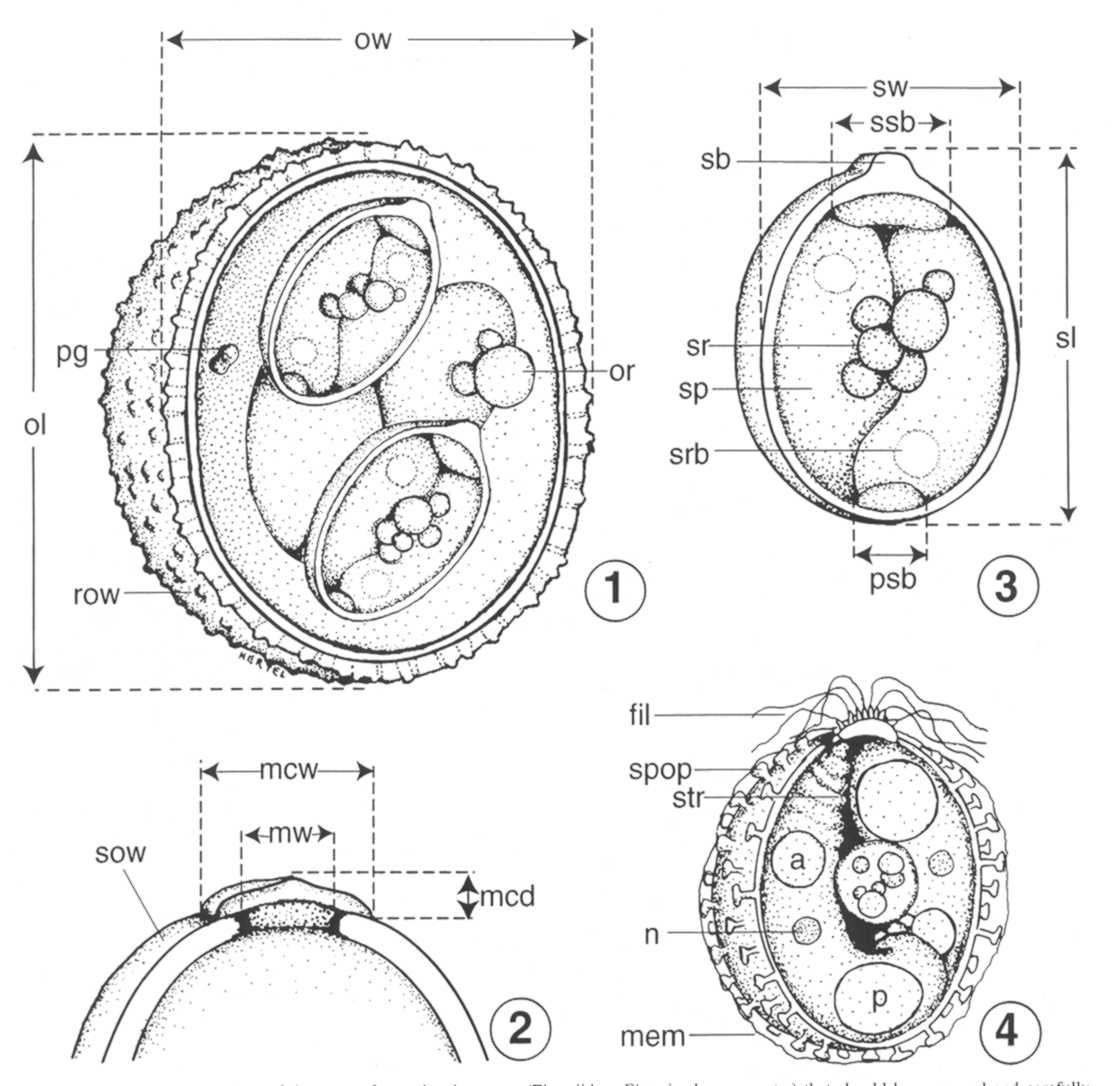
- Use only sporulated oocysts (Fig.1, below) for
mensural
data.
- Supply measurements (means [plus/minus SD] and ranges) of
at least 30-50 sporulated oocysts (100 would be best) to include: oocyst
length (ol), oocyst width (ow), sporocyst length (sl), sporocyst width
(sw), oocyst and sporocyst length : width (L:W) ratios (Figs. 1, 3).
- Note characteristic features of the outer oocyst wall and
any inner layers to include: rough (r) or smooth (s) outer surface
texture
(row, Fig. 1; sow, Fig. 2); spines or conical projections (see
McAllister and Upton, 1989); and relative number of layers and
approximate thickness(es).
- Note presence/absence of the following structures in/on
the sporulated oocyst and, if present, their size, approximate location,
and a description: micropyle (m) and its width (mw, Fig. 2): micropyle
cap (mc), its width and depth (mcw x mcd, Fig. 2); residuum (or), its
diameter and description (or, Fig. 1); polar granule(s) (pg), its/their
size, shape (pg, Fig. 1) or if they attach in a unique manner to the
inner surface of the oocyst wall (see Parker and Duszynski, 1986).
- Note presence/absence of the following structures in/on
the
sporocyst: surface features such as sporopodia (spop, Fig 4); adhering
membranes (mem, Fig. 4); ridges (see Box et al., 1980) or sutures (see
Molnár, 1996); residuum (sr), its diameter and description (sr,
Fig.
3); Stieda body (sb, Fig. 3) and associated filaments (fil, Fig. 4);
substieda body (ssb, Fig. 3) and/or parastieda body (psb, Fig. 3).
- Note presence/absence of the following structures in/on
the sporozoite: refractile body (srb, Fig. 3) and its/their number,
diameter, and shape; nucleus (n, Fig. 4); andother defining features
such as anterior striations (str, Fig. 4), if visible.
- Deposit at least 1 phototype (see Bandoni and Duszynski,
1988) of a sporulated oocyst into an accredited or appropriate
national/regional museum. In the USA, these would include the United
States National Parasite Collection (USNPC), Beltsville, Maryland, or
the Manter Parasitology Laboratory (MPL), Lincoln, Nebraska.
- Provide a composite line drawing with the new species
description that shows all of the structural features that make the
new species unique; this should be drawn exactly to scale using the
mean ol, ow, sl, and sw measurements and include all distinctive
structural features mentioned in the description.
- Be sure that the published manuscript includes at least 1
photomicrograph of a sporulated oocyst and the USNPC, MPL, or other
museum accession number in addition to the composite line drawing.
- Minimally, the new coccidian species should be compared in
detail to the coccidian species that is most structurally similar to it
within the same host genus; however, it would be even better to compare
it to all described species found in the host family to avoid naming a
new species based solely on host species.
- Assuming that the collected sample of oocysts used in
the species description was "pure," (i.e., had only one putative species
[morphotype]), then some oocysts should be preserved in 70% ethanol and
archived in an accredited museum in the event that future workers choose
to amplify and sequence the parasite's DNA (Relman et al., 1996).
- The organ(s) and which part was infected; state if any
organs were examined or whether oocysts were collected only from fecal
material.
- Pathogenicity and histopathological
observations.
Also see Duszynski and Wilber, 1997.
Literature Cited
Bandoni, S.M. and D.W. Duszynski. 1988. A plea for improved
presentation of type material for coccidia. Journal of Parasitology 74:
519-523.
Box, E.D., A.A. Marchiondo, D.W. Duszynski, and C.P. Davis. 1980.
Ultrastructure of Sarcocystsis sporocysts from passerine birds
and opossums: Comments on classification of the genus Isospora.
Journal of Parasitology 66: 68-74.
Couch, L., D.W. Duszynski and E. Nevo. 1993. Coccidia (Apicomplexa),
genetic diversity, and environmental unpredictability of four chromosmal
species of the subterranean superspecies Spalax ehrenbergi
(mole-rat) in Israel. Journal of Parasitology 79: 181-189.
Duszynski, D.W. 1986. Host specificity in the coccidia of small mammals:
Fact or fiction? In Advances in protozoological research. M.
Bereczky (ed.). Symposia Biologica Hungarica, Vol. 33. Akademiai Kiado,
Budapest, Hungary, p. 325-337.
Duszynski, D.W. and G.A Conder. 1977. External factors and
self-regulating mechanisms which may influence the sporulation of
oocysts of the rat coccidium, Eimeria nieschulzi. International
Journal of Parasitology 7: 83-88.
Duszynski, D.W. and S.L. Gardner. 1991. Fixing coccidian oocysts is not
an adequate solution to the problem of preserving protozoan type
material. Journal of Parasitology 77: 52-57.
Frey, J.K., T.L. Yates, D.W. Duszynski, W.L. Gannon and S.L. Gardner.
1992. Designation and curatorial management of type host specimens
(symbiotypes) for new parasite species. Journal of Parasitology 78:
930-932.
Lom, J. and J.R. Arthur. 1989. A guideline for the preparation of
species
descriptions in Myxosporea. Journal of Fish Diseases 12: 151-156.
McAllister, C.T. and S.J. Upton. 1989. The coccidia (Apicomplexa:
Eimeriidae) of testudines, with descriptions of three new species.
Canadian Journal of Zoology 67: 2459-2467.
Molnár, K. 1996. Eimerian infection in the gut of the tube-nosed
goby Proterorhinus marmoratus (Pallas) of the River Danube.
Systematic Parasitology 34: 43-48.
Parker, B.B. and D.W. Duszynski. 1986. Coccidiosis of sandhill cranes
(Grus canadensis) wintering in New Mexico. Journal of Wildlife
Diseases 22: 25-35.
Relman, D.A., T.M. Schmidt, A. Gajadhar, M. Sogin, J. Cross, K. Yoder,
O. Sethabutr and P. Echeverria. 1996. Molecular phylogenetic analysis of
Cyclospora, the human intestinal pathogen, suggests that it is
closely related to Eimeria spp. The Journal of Infectious
Diseases
173: 440-445.
Sheather, A.L. 1923. The detection of intestinal protozoa and mange
parasites by a flotation technique. Journal of Comparative Pathology 36:
266-275.
Upton, S.J., S.L. Gardner and D.W. Duszynski. 1988. The round stingray,
Urolophus halleri (Rajiformes: Dasyatidae), as a host for
Eimeria chollaensis sp. nov. (Apicomplexa: Eimeriidae). Canadian
Journal of Zoology 66: 2049-2052.
Wash, C.D., D.W. Duszynski and T.L. Yates. 1985. Eimerians from diffrent
karyotypes of the Japanese wood mouse (Apodemus spp.), with
descriptions of two new species and a redescription of Eimeria
montgomeryae Lewis and Ball, 1983. Journal of Parasitology 71:
808-814.
Wilber, P.G., B. Hanelt, B. Van Horne and D.W. Duszynski. 1994. Two new
species and temporal changes in the prevalence of eimerians in a
free-living population of Townsend's ground squirrels Spermophilus
townsendii) in Idaho. Journal of Parasitology 80: 251-259.
Wilber, P.G., K. McBee, D.J. Hafner and D.W. Duszynski. 1994. A new
coccidian (Apicomplexa: Eimeriidae) in the northern pocket gopher
(Thomomys talpoides) and a comparison of oocyst survival in hosts
from radon-rich and radon-poor soils. Journal of Wildlife Diseases 30:
359-364.
