The protistan phylum Apicomplexa
Levine,
1970 contains many obligate intracellular parasites and includes such
diverse organisms as coccidia, gregarines, piroplasms, haemogregarines
and the malarias. Members of this large, heterogeneous assemblage are
united, not necessarily by their biology and/or life histories, but by
the presence of a unique "apical complex," composed of polar rings,
rhoptries, micronemes, often a conoid, and other subcellular organelles,
but visualized only by use of an electron microscope.
The coccidia, along with the gregarines,
comprise the class Conoidasida Levine, 1988, characterized by the
presence of a complete, hollow, truncated conoid. While the gregarines
parasitize invertebrates with mature gamonts being extracellular, the
coccidia mostly infect vertebrates and have intracellular gamonts. The
coccidia are separated into four orders, each distinguished by the
presence or absence of various asexual and sexual stages. The largest
order, Eucoccidiorida Lèger & Duboscq, 1910 contains species that
all undergo merogony (asexual), gamogony (sexual) and sporogony (spore
formation) during their life cycle. Members of the family Eimeriidae
Minchin, 1903 all are homoxenous (direct life cycle), with merogony,
gamogony and the formation of oocysts occurring within the same host.
Oocysts then leave the host via the feces, and are unsporulated
(undeveloped, non-infective). The development of a
genetically-determined number of sporocysts and sporozoites within each
oocyst usually occurs outside the host if/when environmental conditions
(oxygen, moisture, temperature) are appropriate.
The genus Eimeria Schneider,
1875, with more than 1300 species described to date, is the largest
apicomplexan genus, and may be the most specious genus of all animal
genera! Sporulated oocysts of Eimeria contain four sporocysts,
each with two sporozoites.
Structure and Life History
An eimerian species was one of the first
protists ever visualized when Antoni van Leeuwenhoek saw what surely
were oocysts of Eimeria steidai (Lindemann, 1895) in the bile of
a rabbit in 1674. Since the oocyst is the stage that leaves the host,
usually in the feces, it is the structure in the life cycle that readily
is available to the veterinarian, wildlife biologist, or parasitologist
who wants to identify the species, often without having to kill the
host. As a result, about 98% of all Eimeria species are known only
from this one life-cycle stage, the sporulated oocyst.
The complete life cycle of a "typical"
Eimeria species is shown in Figure 1.
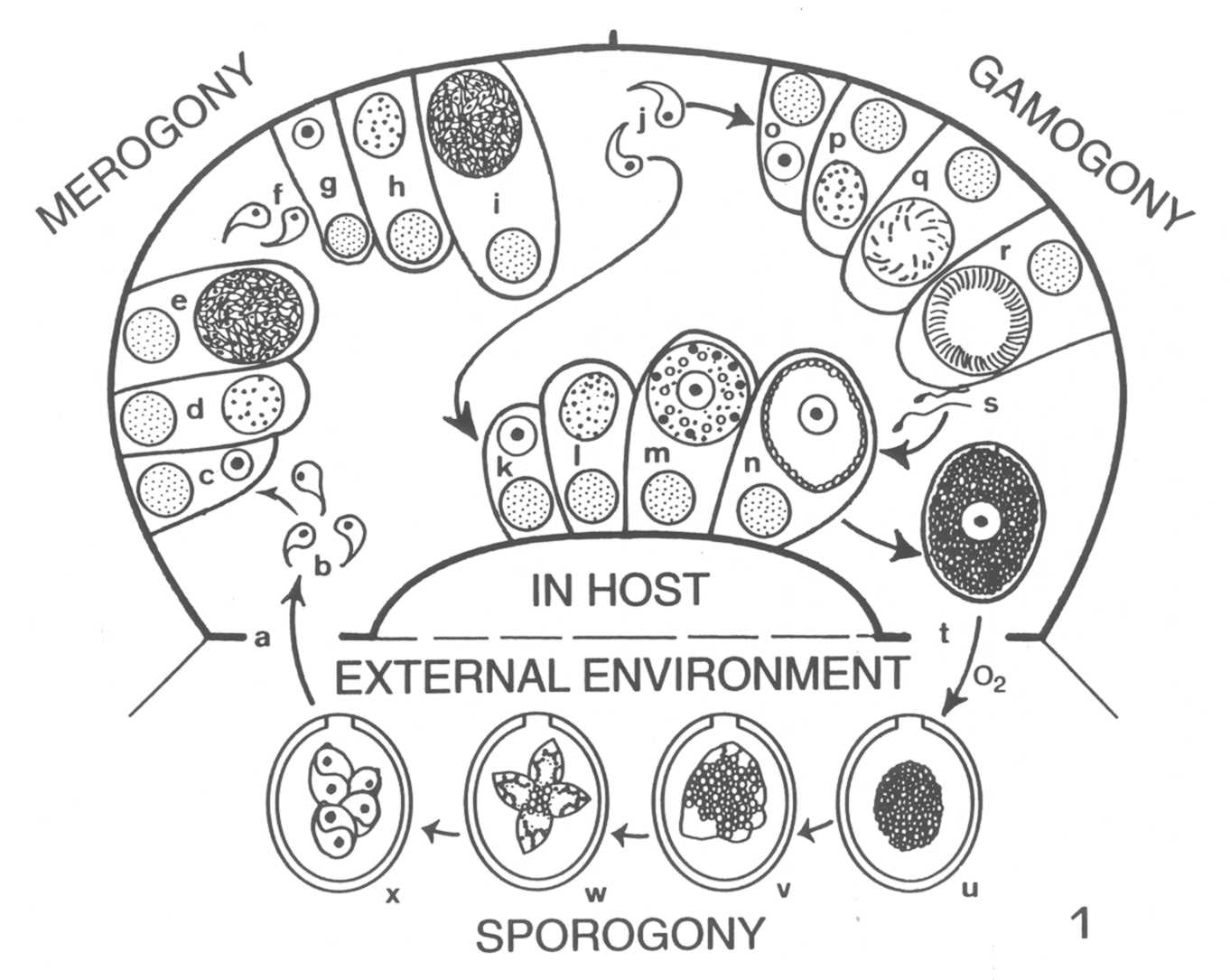
When a sporulated oocyst is ingested (Fig. 1, a) by the proper host, the
sporozoites must first leave the confines of the sporocyst and oocyst
(i.e., they must "excyst") before infection can proceed. Both
mechanical (muscular contractions) and enzymatic digestive (trypsin,
bile salts) processes of the upper gastrointestinal tract of the host
make
the sporocyst and oocyst walls more permeable; eventually, certain parts
of each may be digested, or they may collapse or are broken, releasing
their sporozoites (Fig. 1, b). Once free within the milieu of the
intestine, a sporozoite must penetrate a host epithelial cell before
development can continue (Fig. 1, c). Invasion of the host cell is
complicated, involving a sequential series of steps including
recognition of a host cell, attachment to surface components, formation
of a tight juction, entry into the cell (facilitated by organelles of
the
apical complex), and formation of a parasitophorous vacuole around the
sporozoite (Sam-Yellowe, 1996). Safely inside its parasitophorous
vacuole, the sporozoite initiates merogony (asexual multiple
fission).
During merogony (Fig. 1, c-e), as few as two and up to as many as
100,000 merozoites may be formed by each sporozoite, depending on the
species. Mature merozoites rupture and kill the host cell (Fig. 1, f),
each seeking to penetrate a new epithelial cell to begin merogony again
(Fig. 1, g-i). It is believed that each Eimeria is programmed
genetically for a specific number of merogonous generations
characteristic of that species. For the few species in which the actual
number of generations is known, it varies from two to four generations.
Thus, infections with Eimeria species are self-limiting as
asexual reproduction does not continue indefinitely. Nonetheless,
whatever the number, tremendous biological magnification of the parasite
results from these developmental stages.
When the last generation of merozoites
(Fig. 1, j) enter host epithelial cells, they develop not into
additional meronts, but gamonts. The vast majority develop into
macrogametocytes (macrogamonts) to form uninucleate macrogametes (Fig.
1, k-n), while the remaining merozoites develop into microgametocytes, each
of which will undergo multiple fission to produce thousands of motile,
biflagellated microgametes (Fig. 1, o-r). When they are mature,
microgametes leave their host cell (Fig. 1, s), seek out and penetrate
cells that have a mature macrogamete within (Fig. 1, n), and
fertilization occurs, restoring the diploid (2N) condition. Soon after
fertilization, a delicate membrane forms around the zygote and two
types of wall-forming bodies develop in the cytoplasm; these migrate
toward, and then fuse with, the surface membranes to form the resistant
oocyst wall. When the oocyst wall is fully formed, the oocyst ruptures
from the host cell and leaves the host (Fig. 1, t), usually in the
feces. The mechanisms that regulate if a merozoite will become a macro-
or microgamont, how microgametes find cells with developed macrogametes,
and details of the fertilization process all are topics that warrant
further study.
It has been demonstrated experimentally
that at least a few bird and mammalian Eimeria may form
extraintestinal tissue stages (Carpenter, 1993; Mottalei et al., 1992).
Apparently, sporozoites excyst from oocysts ingested by these host,
infect cells in various places in the body, and become dormant. The
infected host may or may not be the "normal" host for that eimerian
species; if the host with such tissue stages is eaten by the
appropriate host, these dormant sporozoites become active, infect
enterocytes (intestinal epithelial cells), and intitiate their typical
life cycle. It is not known if such a cycle is functional in natural
communities.
The time between when a suitable host
first ingests a sporulated oocyst and when unsporulated oocysts leave
the host in its' feces is termed the prepatent period; during this
interval, which can vary from three to ten days, no oocysts are found in
the feces because only merogony and the beginning of gamogony are
occurring in the host. The time interval during which oocysts are
discharged from an infected host is termed the patent period and lasts
only until all the fertilized and unfertilized macrogametes have been
released from their host cells. Both time periods vary between host and
Eimeria species and are dependent upon many factors including:
eimerian species, size of inoculating dose, number of endogenous stages,
depth within the tissues where merogony, gamogony and fertilization
occur, concurrent infection with other parasites, host age, and
nutritional and immune status of the host.
Once outside the host, the oocyst must
sporulate before it is infective to another host animal (Fig. 1, u-x).
The presence of oxygen, moisture, shade (direct exposure to UV
radiation---sunlight---will kill oocysts quickly) and, generally, a
temperature less than body temperature of the host, are necessary for
oocyst survival. If these conditions are met, the first nuclear division
of the sporoplasm (diploid zygote) within the oocyst is meiotic; all
subsequent cell divisions, which ultimately lead to the formation of
four sporocysts, each containing two sporozoites, are mitotic.
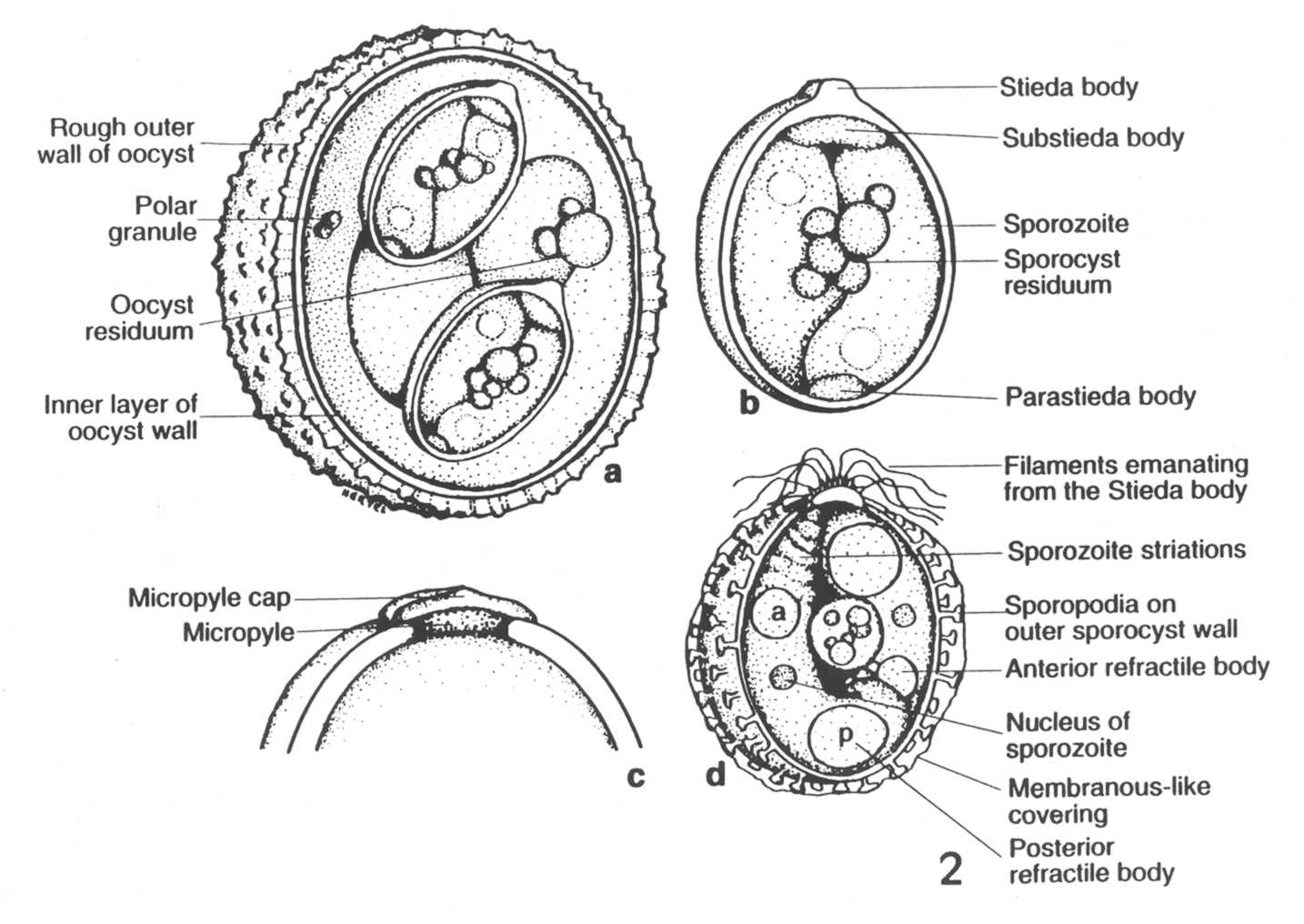
Once the
sporulation process is completed, the fully-formed oocyst (Fig. 2, a, c)
and its sporocysts (Fig. 2, b, d) have a suite of structural characters
that help the experienced taxonomist distinguish one species from the
next. Unfortunately, sometimes sporulated oocysts from different host
species look very nearly identical in size and structure and may not be
easily or reliably differentiated by morphological features alone. Once
sporulation is completed, the oocysts are resistant to environmental
extremes and the sporozoites therein are immediately infective to the
next suitable host animals that may ingest them.
Survival of Oocysts
Moisture, temperature, and direct
exposure to sunlight all influence the ability of oocysts to sporulate
in the external environment, but the interactions of these and other
factors (e.g., mechanical vectors such as invertebrates) are not well
understood. In general, oocysts sporulate more rapidly at higher
temperatures and slower at lower temperatures; exposure to temperatures
less than 10 º C or greater than 50 º C are lethal to
unsporulated oocysts. Between these extremes, the sporulation of oocysts
in a field-collected sample is dependent upon at least the following
factors: the eimerian species, the time and temperature between
collection and arrival of the sample at the lab, the medium in which the
sample was stored, the amount of molecular oxygen available to the
stored oocysts and the concentration of oocysts in the sample. Under
optimal laboratory conditions, sporulation of oocysts from mammals
occurs best between 20-23 º C, but this will vary among vertebrate
classes (Duszynski and Wilber, 1997). Once sporulated, oocysts of some
species remain viable and infective in 2% aqueous potassium dichromate
(kills bacteria, prevents putrification) at 4-5 º C for up to
four years. In their natural external environment, oocysts remain viable
and infective from as little as 49 days up to 86 weeks, dependent upon
the species and the interplay of abiotic and biotic environmental
parameters. See our Techniques for Preservation of Oocysts page:
http://biology.unm.edu/coccidia/techniques.html
Specificity
Eimeria species demonstrate both
site and host specificity, but to somewhat different degrees. The
majority of species, for which endogenous development is known, undergo
development within certain cells of the gastrointestinal tract, but not
all species are found in this location. Eimeria steidai undergoes
develpment in epithelial cells of the bile duct and parenchymal cells of
the liver of rabbits. Other species have been found to develop in cells
of the gall bladder (goat), placenta (hippopotamus), epididymis (elk),
uterus (impala), genitalia of both sexes (hampsters), bile duct
(chamois), liver parenchyma (wallaby), and pyloric antrum (kangaroo)
(Duszynski and Upton, 2001). Once within their specific organ system of
choice, Eimeria species seem to be limited to specific zones
within that system, specific cells within that zone, and specific
locations within those cells. Thus, one species may be found only in the
middle third of the small intestine and another only in the cells of the
cecum. Within their specific region, one species may be found only in
cells at the base of the Crypts of Lieberkühn, a second species in
epithelial cell along the villi, and a third species in endothelial
cells of the lacteals in the villi. Some species devlop below the
striated (microvillus) border of endothelial cells, but above the
nucleus, others below the nucleus, and a few within the nucleus.
The degree of host specificity seems to
vary between host groups; it has been studied best in mammals, and to a
lesser degree in birds, especially domesticated stock/flock animals.
Eimeria species from goats cannot be transmitted to sheep and
vice versa (Lindsay and Todd, 1993), but Eimeria from
cattle (Bos) are found to infect American bison (Bison).
Eimeria species from certain rodents (Sciuridae) seem to cross
host generic boundaries easily (Wilber et al., 1998), while other rodent
species (Muridae) may cross species, but not genus, boundaries (Hnida
and Duszynski, 1999). Similarly, some species from gallinaceous birds
can be transmitted only to congenerics, while others can be
cross-transmitted between genera. One species even has been reported to
cross familial lines, but this seems rare (DeVos, 1970). It also is
known that Eimeria separata Becker and Hall, 1931 from rats will
infect certain genetic strains of mice and that genetically altered or
immunosuppressed mammals are susceptible to infection with
Eimeria species to which they otherwise might be naturally
refractory. Thus, numerous biotic interactions, particularly the genome
of both parasite and host, must contribute to the host specificity, or
lack thereof, attributed to each Eimeria species.
Literature Cited
DeVos, A.J. 1970. Studies on the host
range of Eimeria chinchillae DeVos and Van der Westhuizen, 1968.
Ondersteeport Journal of Veterinary Research 37: 29-36.
Duszynski, D.W. and P.G. Wilber. 1997. A guideline for the preparation
of species descriptions in the Eimeriidae. Journal of Parasitology 83:
333-336.
Duszynski, D.W. and S.J. Upton. 2001. Enteric protozoans: Cyclospora, Eimeria, Isospora and Cryptosporidium
(Cryptosporidiidae) spp. Chapter 16, pp. 416-459,In, Parasitic Diseases of Wild Mammals, 2nd ed. (W.M. Samuel, M.J.
Pybus, A.A. Kocan, eds.) Iowa State University Press, Ames, IA.
Hnida, J.A. and D.W. Duszynski. 1999. Cross-transmission studies with
Eimeria arizonensis, E. arizonensis-like oocysts and E.
langebarteli: host specificity within the Muridae and other
rodents. Journal of Parasitology 85: 873-877.
Lindsay, D.S. and K.S. Todd, Jr. 1993. Coccidia of mammals. In,
Parasitic protozoa. Vol. 4. pp. 89-131. Academic Press, Inc., New
York.
Sam-Yellowe, T.Y. 1996. Rhoptry organelles of the Apicomplexa: Their
role in host cell invasion and intracellular survival. Parasitology
Today 12: 308-315.
Wilber, P.G., D.W. Duszynski, S.J. Upton, R.S. Seville and J.O. Corliss.
1998. A revision of the taxomnomy and nomenclature of the eimerians
(Apicomplexa: Eimeriidae) from rodents in the tribe marmotini
(Sciuridae). Systematic Parasitology 39: 113-135.

